With nighttime temperatures forecasted in the 40s next week, it’s no surprise that the time for influenza vaccination is upon us. And since this year’s seasonal flu coincides with an ongoing COVID-19 pandemic that is worse in America than in almost any other developed country, and because influenza vaccination will save lives and reduce symptoms that might be confused with COVID-19, CDC has details on new recommendations for vaccination. Here are the highlights of new information about the flu shot:
Everyone aged 6 months and above should receive the influenza vaccine.
Anticipate about two weeks from immunization to protection from the influenza virus.
The timing of your shot isn’t critical. The duration of immunity from a flu shot is about four months, so the CDC recommends immunization before Halloween if possible in order to provide maximum protection through the heart of the flu season.
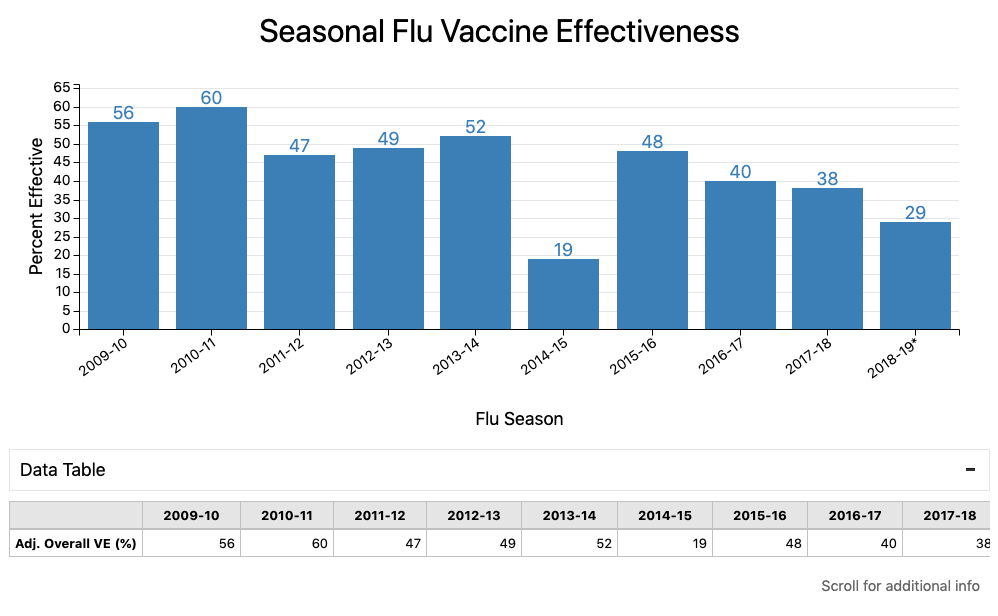
Expect the vaccine to cut your risk of infection by about half, give or take:
As always, the composition of the seasonal influenza vaccine is an educated guess based on trends in detected circulating viruses. The vaccine historically includes either three (“trivalent”) or four (“quadrivalent”) strains of virus. Each influenza A virus expresses a version of a sugary protein called hemagglutinin, or “H,” and a version of an enzyme called neuraminidase, or “N.” This year, in case you’re interested, trivalent egg-based vaccines contain:
Influenza A/Guangdong-Maonan/SWL1536/2019 (H1N1)pdm09 (a version of the virus implicated in the 2009 H1N1 Pandemic)
Influenza A/Hong Kong/2671/2019 (H3N2)
B/Washington/02/2019 (B/Victoria; a strain that accounted for the vast majority of influenza B infections last year)
Quadrivalent (four-component) egg-based vaccines contain those three viruses in addition to influenza B/Phuket/3073/2013(Yamagata)
Non-egg-based vaccines contain:
A different H1N1 called influenza A/Hawaii/70/2019(H1N1)pdm09
A different H3N2 called A/Hong Kong/45/2019(H3N2)
The same B/Washington/02/2019(B/Victoria)
The same B/Phuket/3073/2013(Yamagata)
As you can tell, many versions of the influenza vaccine are available (and two new versions for folks aged 65 and up were recently approved), but the most important choice is not which vaccine to receive, but whether to get vaccinated. If you have questions about which vaccine is best for you, we encourage that you talk to your doctor or other health care professional.
Common reasons to avoid a flu vaccination are having a fever that day, allergies to certain foods or ingredients like eggs or latex, or a previous serious reaction like swelling or seizure. Feeling a little sore after last year’s injection is not a reason to skip this year’s.
Because remember: It is impossible to get influenza from the vaccination. The influenza vaccine contains only small amounts of either a protein that stimulates the immune system or an inactivated virus that is not capable of infecting anyone. In randomized, blinded trials (paywall) where some people got flu shots and others got only saline placebo shots, the only differences in symptoms was increased soreness in the arm and redness at the injection site in those people who got a flu shot. There were no differences between the groups in body aches, fever, cough, runny nose, or sore throat.
In related news, since rates of routine childhood immunization have fallen during the pandemic, new rules from the Department of Health and Human Services now state that pharmacists nationwide will be able to administer routine childhood vaccines–that is, FDA-approved vaccines recommended by the CDC’s Advisory Committee on Immunization Practices for youth ages 3 to 18–during the COVID-19 pandemic. Needless to say, this is controversial.
For other questions about influenza vaccination, contact us or go to www.cdc.gov/flu/. Now go get your shot!
As the Medical Director of the Kansas Business Group on Health I’m sometimes asked to weigh in on topics that might affect employers or employees. This was a reprint of a blog post from KBGH.